A microglial cell, labelled in green, contacts and attacks a myelinated axon (in red). In the presence of the pHERV-W envelope protein, this interaction leads to axonal injury. The blue structures are cell nuclei. Credit: HHU / Joel Gruchot / Patrick Küry
It is surprising that only about 2% of human DNA encodes the 20,000 or so genes we all have. The other 98% used to be called junk DNA.
About 8% of your DNA is made up of Endogenous retroviruses (ERVs) that have been picked up during evolution and most of which have been inactivated and can indeed be regarded as junk. Some of these old viruses that became part of human DNA remain fully functional, can be activated; they are implicated in disease ranging from Multiple Sclerosis (MS), to cancer, to schizophrenia and ALS (motor neuron disease).
The best documented ERV is the one that affects some people with MS, it is called HERV-W (the H is for Human). Only in the presence of a protein encoded by this virus can the microglia cells attack the myelin layer on axons. In this kind of MS, if you could switch off the HERV-W virus you would solve the remyelination problem.
The thing to remember is that MS is a family of conditions and HERV-W may only be relevant to specific sub-types. The recent research (see below) produced the image at the start of today’s post, where we actually see the microglia (green) mistakenly attacking the healthy myelin on axons (red).
As outlined by first author Dr. David Kremer, the envelope (ENV) protein of the pathogenic human endogenous retrovirus type W (pHERV-W) was found to be a major contributor to nerve damage in MS. In collaboration with research teams in the U.S. and Canada, the authors demonstrated that the ENV protein drives CNS resident microglial cells to contact and damage myelinated axons.
There is a broad repertoire of immunomodulatory drugs that effectively treat the inflammatory aspects of relapsing multiple sclerosis (MS). However, axonal degeneration, which occurs mainly in progressive MS, is still not understood and cannot be treated pharmaceutically. As it is the major factor contributing to clinical disability in MS, it represents an unmet clinical need. A recently completed phase IIb study has demonstrated that anti-pathogenic human endogenous retrovirus type W (pHERV-W) envelope protein (ENV) treatment results in a significant decrease of neurodegenerative brain atrophy in treated MS patients. For these results, the work presented here offers an explanation by demonstrating that, via myeloid cells, pHERV-W ENV directly harms axons.
Axonal degeneration is central to clinical disability and disease progression in multiple sclerosis (MS). Myeloid cells such as brain-resident microglia and blood-borne monocytes are thought to be critically involved in this degenerative process. However, the exact underlying mechanisms have still not been clarified. We have previously demonstrated that human endogenous retrovirus type W (HERV-W) negatively affects oligodendroglial precursor cell (OPC) differentiation and remyelination via its envelope protein pathogenic HERV-W (pHERV-W) ENV (formerly MS-associated retrovirus [MSRV]-ENV). In this current study, we investigated whether pHERV-W ENV also plays a role in axonal injury in MS. We found that in MS lesions, pHERV-W ENV is present in myeloid cells associated with axons. Focusing on progressive disease stages, we could then demonstrate that pHERV-W ENV induces a degenerative phenotype in microglial cells, driving them toward a close spatial association with myelinated axons. Moreover, in pHERV-W ENV-stimulated myelinated cocultures, microglia were found to structurally damage myelinated axons. Taken together, our data suggest that pHERV-W ENV-mediated microglial polarization contributes to neurodegeneration in MS. Thus, this analysis provides a neurobiological rationale for a recently completed clinical study in MS patients showing that antibody-mediated neutralization of pHERV-W ENV exerts neuroprotective effects.
Relapsing-Remitting Multiple Sclerosis (RRMS)
Most MS starts out as so-called Relapsing-Remitting Multiple Sclerosis (RRMS) and so is the focus of much research. An antibody called GNbAC1 has been developed to specifically target the protein MSRV-Env that is produced by the old human endogenous retrovirus type W.
In vitro and in vivo studies showed that GNbAC1 neutralizes MSRV-Env, reducing the inflammatory response and allowing the remyelination repair process to restart.
I think this is an excellent example of how to translate complicated science into a practical therapy. I just hate to think how much money this therapy will cost.
Or just Antivirals?
I did wonder about a less expensive therapy to block the MSRV-Env protein from activating microglia to destroy myelin. Why not use a relatively cheap antiviral drug to dampen the virus itself, so it does not make the harmful protein?
Unlike most antibiotics, antiviral drugs do not destroy their target pathogen; instead they inhibit their development.
Antiviral drugs normally have to be developed to target a specific virus, but you might just get lucky with an existing drug.
In the case of HIV, a combination of three drugs is used TDF (tenofovir), EFV (efavirenz) and either 3TC (lamivudine) or FTC (emtricitabine). This therapy has been hugely successful.
The anti-herpes antivirals include valacyclovir (Valtrex), famciclovir (Famvir), and acyclovir (Zovirax).
In the case of Multiple Sclerosis, I did find a study that used acyclovir. It did not cure the condition, but it did significantly reduce exacerbations.
I am afraid nobody seems to want a cheap drug for MS, when the other only partially effective ones can cost $50,000 a year. Acyclovir is much more expensive in the US than elsewhere but nothing like the price of the new MS drugs.
It may of course be a coincidence that Acyclovir reduces exacerbations in MS and may involve an entirely different mechanism.
Human endogenous retroviruses (HERVs) beyond MS
Drugs for MS are a huge business for pharmaceutical companies and this is why the research is advanced.
HERVs have been implicated in ALS (motor neuron disease) and schizophrenia. There is even some research on HERVs and autism.
It is usually the Herpes virus that gets mentioned in the context of autism. It is probably one of hundreds of possible triggers that, when combined with other “hits” and genetic predispositions, may lead to autism.
Any virus can affect gene expression and so any virus has the potential to cause harm to a developing brain. This is often all "autism" is, the result of some damage at a critical point in the brain's development. That same event in a teenager does no long term harm.
“We’re not saying that HSV-2 is responsible for infecting the [fetal] brain and causing autism,” stresses senior author Ian Lipkin, an infectious disease expert and epidemiologist at Columbia. Indeed, fetal infection with HSV-2 is so serious that it frequently leads to miscarriages or stillbirths. Rather, Lipkin suspects that HSV-2 is just one among many environmental insults that, when they arrive at a vulnerable point in fetal development in women predisposed to damaging reactions, may trigger ASD in the fetus. That idea comports with a body of previous work, like this Swedish study that found that the hospitalization of a woman for any kind of infection during pregnancy increased the risk of the baby developing ASD by 30%.
Some scientists are skeptical that inflammatory molecules alone could be responsible, in part because of the big changes in brain structure that arise in autistic children in the first 2 years of life, just as symptoms of ASD emerge. For instance, a study published in Nature last week documents abnormal overgrowth of the surface of the brain in 6- to 12-month-old babies who go on to be diagnosed with ASD.
What if the missing 'environmental' factor in some of our deadliest neurological diseases were really written in our genome? Researchers explain how viruses ended up in our DNA -- and what puts them in the frame in unsolved diseases like multiple sclerosis.
The enemy within
A whopping 8% of our DNA comes from viruses. Specifically, ones called retroviruses -- not because they're old, but because they reverse the normal process of reading DNA to write themselves into their host's genome.
Retroviruses are old though: they began merging with our earliest, primordial ancestors millions of years ago. Over the millennia, most of their remnants in our DNA -- known as human endogenous retroviruses or HERVs -- have been silenced by mutations. Others, which had evolved to fend off rival viruses, formed the prototypical immune system and to this day protect us from infection.
However, HERVs might also be the missing causative link in major 'unsolved' neurological diseases.
"HERVs have been implicated in the onset and progression of multiple sclerosis [MS], amyotrophic lateral sclerosis [ALS] and schizophrenia [SCZ]," says senior author Prof. Patrick Kuery. "Dormant HERVs can be reactivated by environmental factors such as inflammation, mutations, drugs, or infection with other viruses, so could provide a mechanism for their well-established epidemiological link to these disorders."
Full paper: -
Human endogenous retroviruses (HERVs) are ancient retroviral elements, which invaded the human germ line several million years ago. Subsequent retrotransposition events amplified these sequences, resulting in approximately 8% of the human genome being composed of HERV sequences today. These genetic elements, normally dormant within human genomes, can be (re)-activated by environmental factors such as infections with other viruses, leading to the expression of viral proteins and, in some instances, even to viral particle production. Several studies have shown that the expression of these retroviral elements correlates with the onset and progression of neurological diseases such as multiple sclerosis (MS) and amyotrophic lateral sclerosis (ALS). Further studies provided evidence on additional roles for HERVs in schizophrenia (SCZ). Since these diseases are still not well understood, HERVs might constitute a new category of pathogenic components that could significantly change our understanding of these pathologies. Moreover, knowledge about their mode of action might also help to develop novel and more powerful approaches for the treatment of these complex diseases. Therefore, the main scope of this review is a description of the current knowledge on the involvement of HERV-W and HERV-K in neurological disease specifically focusing on the effects they exert on neural cells of the central nervous system.
Importantly, several studies were able to show that inflammation plays a major role in HERV activation
SCZ is a complex neuropsychiatric disorder characterized by a variety of cognitive, emotional, and perceptual disturbances. Pathophysiologically, SCZ features decreased brain volume, loss of myelin, and altered astrocyte function (Archer, 2010). In contrast to MS and ALS, both HERV-W and HERV-K have been weakly linked to SCZ based on PCR amplification from CSF and post-mortem brains as well as on protein antigenemia (Yolken et al., 2000; Karlsson et al., 2001; Frank et al., 2005; Perron et al., 2008), while another study revealed upregulation of HERV-W ENV transcripts in plasma samples of SCZ patients (Huang et al., 2011). Moreover, a new study provides evidence that, in early stages of this disease, HERV-K methylation in peripheral blood is reduced (Mak et al., 2019). Of note, these observations contradict an earlier report suggesting that HERV-W expression is reduced in SCZ patients (Weis et al., 2007). The disparity between these reports may reflect different experimental approaches or a differential use of anti-psychotic medications in SCZ patients.
We here present collected evidence that endogenous retroviral elements acting either as viral particles or via their proteins influence neural cells in the context of degenerative CNS diseases. Once thought to be primarily involved in cell transformation (Grabski et al., 2019) and inflammation (Perron and Lang, 2010), emerging data suggests a direct role of these elements in glial and neuronal injury, which in fact goes beyond previous descriptions on the activity of a gliotoxin (Menard et al., 1998). In light of additional observations on the role of ERVs in regulating stem cell potential and fate acquisition (Gautam et al., 2017), the findings describing impacts on committed or mature cells of the CNS are probably not too surprising but warrant future investigations, even more so since neural stem cells are also involved in brain pathology and regeneration. Moreover, the currently still unmet clinical need to effectively treat neurodegeneration necessitates novel therapeutic approaches. Whether similar mechanisms also apply to activation of transposable elements implicated in, for example, chronic fatigue syndrome (CFS; Almenar-Perez et al., 2019) and to what degree currently used neutralizing antibodies can be exploited in order to prevent neural cell activation and/or neurodegeneration needs to be elucidated in the future. In this regard, it remains to be shown whether HERV-employed signaling pathways and epigenetic silencing mechanisms can be used for biomedical translation.

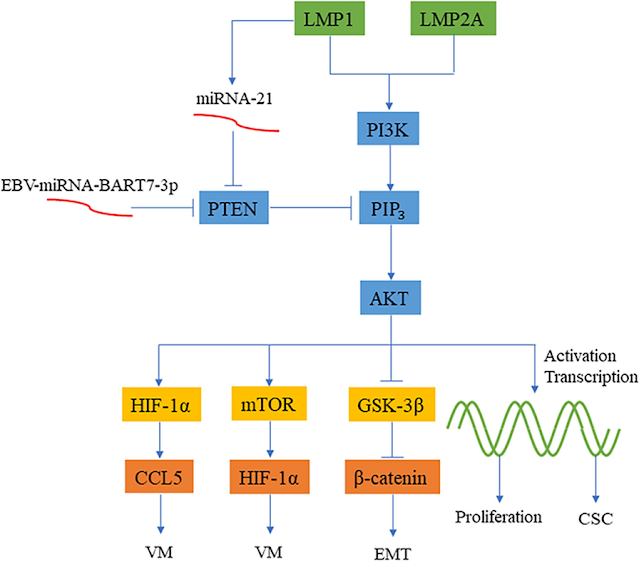
Figure 1 HERV-mediated effects on neural cells. This illustration summarizes origin and observed molecular effects of HERW-W and HERV-K on cells of the central nervous system. Arrow starting points indicate cellular sources of HERV particles or proteins (red dots), whereas arrowheads point to influenced cell types. Modulated processes are shown in gray boxes, and regulated molecules are highlighted in red next to each cell type. The question mark next to TDP-43 refers to its postulated regulation in neurons. Whether microglia and astroglia respond to HERVs in an auto- and/or paracrine way and whether neurons react to internal and/or extracellular HERVs remains to be shown. OPCs: oligodendroglial progenitor cells; NO: nitric oxide; CRP: C-reactive protein; BDNF: brain-derived neurotrophic factor; DRD3: dopamine receptor D3; TRPC3: short transient receptor potential channel 3; DISC1: disrupted in schizophrenia 1; TDP-43: TAR DNA-binding protein 43.
HERVs, retroviral sequences integrated into the genome during evolution, are now known to represent 8% of the human genome.
These were recently shown to comprise copies that retain potential to express retroviral proteins or particles, and can be abnormally expressed in autoimmune, neurodegenerative, chronic inflammatory diseases, and cancer.
Environmental factors such as specific viral infections were shown to potently activate HERVs under tissue-specific and temporal conditions.
Of several diseases in which abnormal activation and expression of HERV proteins have been reported, studies over recent decades have led to a proof of concept that HERVs play a key role in the pathogenesis of MS and ALS.
HERV-W and HERV-K Env proteins induce pathogenic effects in vitro and in vivo that are relevant to the pathognomonic features of these diseases.
These endogenous retroviruses are potential novel therapeutic targets that are now being addressed with innovative therapeutic strategies in clinical trials.
The causes of multiple sclerosis and amyotrophic lateral sclerosis have long remained elusive. A new category of pathogenic components, normally dormant within human genomes, has been identified: human endogenous retroviruses (HERVs). These represent ∼8% of the human genome, and environmental factors have reproducibly been shown to trigger their expression. The resulting production of envelope (Env) proteins from HERV-W and HERV-K appears to engage pathophysiological pathways leading to the pathognomonic features of MS and ALS, respectively. Pathogenic HERV elements may thus provide a missing link in understanding these complex diseases. Moreover, their neutralization may represent a promising strategy to establish novel and more powerful therapeutic approaches.
Results
The percentage of HERV-H and HERV-W positive samples was higher among ASD patients compared to HCs, while HERV-K was similarly represented and HERV-E virtually absent in both groups. The quantitative evaluation shows that HERV-H and HERV-W are differentially expressed in the two groups, with HERV-H being more abundantly expressed and, conversely, HERV-W, having lower abundance, in PBMCs from ASDs compared to healthy controls. PMBCs from ASDs also showed an increased potential to up-regulate HERV-H expression upon stimulation in culture, unlike HCs. Furthermore we report a negative correlation between expression levels of HERV-H and age among ASD patients and a statistically significant higher expression in ASD patients with Severe score in Communication and Motor Psychoeducational Profile-3.
Conclusions
Specific HERV families have a distinctive expression profile in ASD patients compared to HCs. We propose that HERV-H expression be explored in larger samples of individuals with autism spectrum in order to determine its utility as a novel biological trait of this complex disorder.
Recent studies suggest that autism spectrum disorders (ASD) result from interactions between genetic and environmental factors, whose possible links could be represented by epigenetic mechanisms. Here, we investigated the transcriptional activity of three human endogenous retrovirus (HERV) families, in peripheral blood mononuclear cells (PBMCs) from Albanian ASD children, by quantitative real-time PCR. We aimed to confirm the different expression profile already found in Italian ASD children, and to highlight any social and family health condition emerging from information gathered through a questionnaire, to be included among environmental risk factors. The presence of increased HERV-H transcriptional activity in all autistic patients could be understood as a constant epigenetic imprinting of the disease, potentially useful for early diagnosis and for the development of effective novel therapeutic strategies.
Overall, the data obtained in the present study lead us to further support the hypothesis that HERV transcriptional activity is influenced by all the factors mentioned above. Additional work is required to determine if HERV-H expression could be proposed as a biological marker, useful for early detection of children at high risk for ASD, before the appearance of clinical symptoms and for the development of effective new therapeutic strategies. To this end, an in-depth characterization of the potential role of HERV-H in ASD is the major objective of a study currently in progress in murine models. Currently, up to 2% of children worldwide are estimated to be diagnosed with an ASD (Pedersen et al., 2014) and the consistent increment in the prevalence of ASD is considered a pressing challenge for the global public health system. Because children represent more than a third of the Albanian population (Albanian Institute of Statistics 2011) autism is a serious socio-economic problem and its early diagnosis could represent a significant improvement in the treatment of the disease. In fact, if the autistic condition is diagnosed early, a growing repertoire of evidence-based therapies can be applied to give children the best possible chance of life.
Autism Spectrum Disorder (ASD) is a neurodevelopmental disorder that reveals itself by social communication problems, restrictive/repetitive behavior, and language impairment. ASD is a growing problem in the USA and in the world with no commonly-accepted etiology resulting in the absence of effective methods of treatment. Based on more than 80 scientific publications we are proposing the following understanding of ASD: it is a genetic disorder, in which some changes in DNA are resulting from a congenital mother to fetus transmitted infection and maternal immune activation. The infections and maternal immune activation result in oxidative stress and production of pro-inflammatory cytokines and other mediators. Based on this understanding, we developed a method of long-term etiotropic and pathogenetic therapy tailored to major chronic/latent infections, inflammation and immune system aberration. We present six cases of ASD treatment, which included the antiviral medication Valacyclovir and five nutritional supplements. The presented results are based on five cycles of treatment continued for 5 months. In all six cases the treatment resulted in social communication skills and behavioral improvements well as positive changes in the physical and psychological conditions. These improvements covariated with a tendency to normalization of blood and immune parameters. Social communication skills, behavioral, physical and psychological improvements also positively affected parents whose subjected quality of life increased over course of the treatment. According to parents of these children, the proposed treatment had superior efficacy compared to other types of treatment that their children underwent before.
A 4-month course of the oral antiviral agent valacyclovir boosted cognition in herpes simplex virus-1–seropositive patients with bipolar disorder and cognitive impairment in a randomized, double-blind placebo-controlled clinical trial.
Objective
To test our hypothesis that valacyclovir, an antiherpes virus–specific medication, added to antipsychotics (APs) would improve cognitive performance and psychopathology among schizophrenia subjects exposed to neurotropic herpes simplex virus, type 1 (HSV1).
Methods
Using a double-blind placebo-controlled design, we randomized 24 HSV1-seropositive schizophrenia subjects to receive either valacyclovir (n = 12) or placebo (n = 12) for 18 weeks in addition to stable doses of APs. Valacyclovir dose was stabilized at 1.5 g twice daily orally. At each visit, subjects were evaluated for severity of psychopathology and side effects using standardized scales and a study-specific semistructured checklist. A computerized neurocognitive battery validated on both schizophrenia and healthy subjects was administered at baseline and follow-up. Intent-to-treat analysis, using linear regression models that included all randomized subjects, were used to examine differential changes in cognition and psychopathology scores over 18 weeks between valacyclovir and placebo, accounting for placebo response.
Results
Valacyclovir group improved in verbal memory, working memory, and visual object learning compared with placebo group. The effect sizes (Cohen’s d) were 0.79 for working memory, 1.14 for immediate verbal memory, and 0.97 for the visual object learning. Psychotic symptom severity did not improve.
Conclusions
Supplemental valacyclovir may alleviate impairments in cognitive domains that are often observed in schizophrenia but not psychotic symptoms in those exposed to HSV1. If replicated, this approach could provide a novel strategy to treat cognitive impairments in a subgroup of schizophrenia subjects who can be reliably identified using a blood test.
Conclusion
There is a great deal going on in the world of MS research and if you have MS you might as well consider becoming an early adopter.
As expected, the research on how these old viruses, that should be dormant in our DNA, might play a role in autism is not very advanced.
Some people with autism do take antiviral drugs and I think their caregivers think this relates to a virus they have acquired recently or comes from the mother. Perhaps it is an unidentified virus from that 8% of your DNA that has become activated?
In MS the story is complex but now we know for sure what the virus is, where it came from and what it does. You can defeat it with a tailor-made antibody called GNbAC1 or perhaps just beat it down a little with the common antiviral drug Acyclovir.
Note that antiviral drugs each only have an effect on certain types of virus.
Do HERVs really materially affect some people with autism, and its big brothers bipolar and schizophrenia? There is some limited evidence that they may.
People who report that their children with autism do indeed improve on an antiviral drug are unlikely to ever know which virus was the problem and it may not be the one they thought it was, but it is not a crazy idea. If it reduces the symptoms of autism without causing troubling side effects, why not? It is going to work for most autism? Probably not.
For people with Multiple Sclerosis (MS) the science is clear and unambiguous, you need to wipe out the protein called MSRV-Env.
As far as this blog is concerned, we already covered antibiotics in depth.
and today we covered antivirals. These are the “anti- drugs” that our reader Tanya referred to as not being useful in her case of autism; I think she will be in the majority. You have to treat your “minority” case of autism, which is what makes it difficult.
Almost every common autism treatment strategy is misrepresented as a wonder therapy; that is how you sell books, supplements, lab tests and even now I see expensive "training" courses. The reality is somewhat messy and less convenient, but if you read the science great progress does seem to be possible in many cases.