Stop the
Noise!
Conventional wisdom, even among
enlightened neurologists like Manuel Casanova, is that you cannot medically
treat the sensory issues that occur in neurological conditions like autism,
bipolar and schizophrenia.
This blog is very much driven by the
peer-reviewed literature, but very often seems to comes up with alternative interpretations
to what the doctors will say. Today is
another of those days.
I do tell people that you can very
easily get things 100% back to front when developing personalized/precision
medicine. The general idea was correct,
but the effect was the exact opposite to what was hoped for. This is not a failure; this is a learning
experience. Today we see that what works
in schizophrenia is the exact opposite of what works in bipolar. I do like to include schizophrenia and
bipolar in my autism posts, because there is a big overlap between them and the
broad umbrella of dysfunctions found in autism.
Sensory problems are very common in
autism, bipolar and schizophrenia.
This post is mainly about issues with
sound. Vision is closely related. Smell,
taste and texture may be less closely related.
Sound/Hearing issues in autism
Very
often young children with autism do not respond to their name, or some other
sounds; the natural first step is to check their hearing. The majority of the time, their hearing turns
out to be perfect.
As
the child gets older and struggles with sounds like a baby crying, or a dog
barking, parents may begin to feel their child’s hearing is too good!
The medical terms
Hyperacusis is a disorder in loudness perception and should mean you hear
sounds too loudly. The opposite term is
hypoacusis and in the medical jargon it means you are going deaf, rather than
having a volume perception problem
Tinnitus is hearing sounds that do not exist, but there are many possible
causes.
Misophonia means hatred of sound,
but those hated sounds are often very specific repeated human sounds like noisy
eating, chewing, sniffing, coughing or machine-made sounds like a noisy clock
ticking, or even a leaf blower.
There does appear to be a visual equivalent of sound
Misophonia.
For some people, visual triggers can cause a similar reaction. This
might happen if you see someone:
- wagging their legs or feet (foot flapping)
- rubbing their nose or picking at their finger
nails
- twirling their hair or pen
- chewing
gum
Some people suffer from a combination of sound
disorders. Many people with tinnitus
also suffer from Misophonia.
I think many people with autism are affected by a combination
of Hyperacusis and Misophonia.
It seems that many people with Asperger’s suffer from
hyperacusis, a substantial minority experience tinnitus. Almost all who suffer
tinnitus also experience hyperacusis.
I think it might be hard to know if a person with severe
autism and ID had tinnitus.
Tinnitus and hyperacusis in autism spectrum
disorders with emphasis on high functioning individuals diagnosed with
Asperger's Syndrome
Objectives: To evaluate the prevalence of
tinnitus and hyperacusis in individuals with Asperger's Syndrome (AS).
Methods: A home-developed case-history survey
and three item-weighted questionnaires: Tinnitus Reaction Questionnaire (TRQ),
Tinnitus Handicap Inventory (THI), and the Hyperacusis Questionnaire (HQ) were
employed. These tools categorize the subjective response to tinnitus and
hyperacusis. The research tools were mailed to a mailing list of individuals
with Asperger's Syndrome.
Results: A total of 55 subjects diagnosed with
AS were included in the analysis (15.5% response rate). Sixty-nine percent of all respondents (38/55)
reported hyperacusis with an average HQ score of 20.7. Furthermore, 35% (19/55) reported perceiving
tinnitus with average scores of 27 for the TRQ and 23 for the THI. Thirty-one percent (17/55)
reported both hyperacusis and tinnitus. The prevalence of hyperacusis in
the AS respondents remained relatively constant across age groups.
Conclusions: Hyperacusis and tinnitus are more
prevalent in the ASD population subgroup diagnosed with AS under DSM-IV
criteria than in the general public. Hyperacusis also appears to be more
prevalent in the AS population than in the ASD population at large. Future
research is warranted to provide insight into the possible correlation between
tinnitus and hyperacusis symptoms and the abnormal social interactions observed
in this group.
All three terms are just observation diagnoses, they do not
tell you what is the underlying biological cause. In this blog we are interested in the
underlying biology, because the goal is to find an effective treatment.
Hearing issues are common comorbities of well-known medical
conditions; for example, people with type 1 diabetes may well suffer from
tinnitus and hypoacusis.
Schematic block diagram of mechanisms that produce
misophonia, hyperacusis, tinnitus, polycusis, and other false auditory
percepts. Afferents from the cochlea, saccule, somesthetic pathways, and
visceral sensory pathways contribute to processing in auditory lemniscal
pathways. Modular thalamocortical processing is hypothesized to contribute (1)
a common component to comorbid features of hyperacusis and tinnitus, (2) a
component that produces unique features of tinnitus, and (3) component(s) for
other false auditory perceptions. A parallel, interoceptive, and affective
network produces the aversion, annoyance, fear, and pain-like features that may
be associated with hyperacusis and misophonia
Source: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6453992/
The research terms
The medical world is often rather short of enough descriptive
words, just think about all those people with totally different biological
conditions all being diagnosed with “autism”.
A really useful term you will find in the research is sensory
gating.
Sensory gating is a process by which
irrelevant stimuli are separated from meaningful ones. Imagine the boy with Asperger’s sitting in a
private room taking his important exams.
He is alone with the invigilator and maybe a clock on the wall. The clock might be making a ticking sound or
the invigilator might be chewing gum.
All this clever boy has to do is to concentrate on the exam and show how
smart he is. The noisy clock, or the
chewing sound, should be irrelevant, but instead the boy cannot filter out
these sounds and ignore them.
I had exactly this case put to me at an autism conference by
a concerned Grandfather, whose clever grandson failed his important exams.
You can actually measure sensory gating using headphones to
provide the annoying repetitive sound and an EEG to measure how the person’s
brain responds. The first sound should
trigger the brain’s response, but when the sound keeps repeating the response
should fade away. The person has learned
to filter out the annoying but irrelevant sound.
Imagine you are in a storm and the rain is beating down on a
glass roof or windows. The first sound
alerts you to the storm. Did you leave
the upstairs window open? Perhaps you were drying something outside? You might have to take some urgent action, so
you want an alarm bell to go off in your head.
Panic over, you can then just ignore the sound of the rain and before
you know it the storm is over.
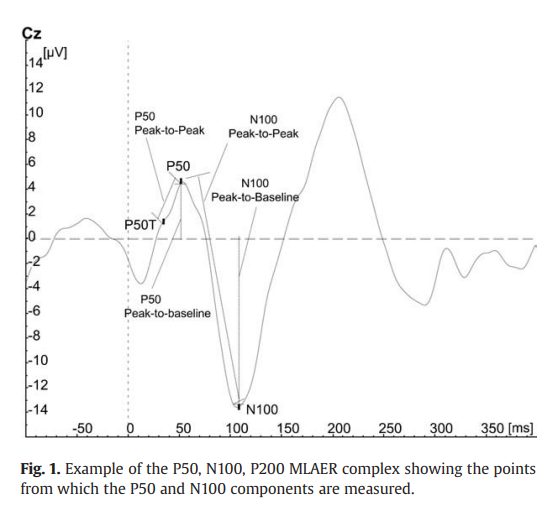
There are different types of sensory gating, the most well
studied is called P50.
People with schizophrenia often have deficits in gating
the neuronal response of the P50 wave, which is why P50 is the
most widespread method of diagnosis. The test is conducted through having the
patients hear two uniform sounds with an interval of 500 milliseconds. While
the patients are hearing the sound, an EEG cap is used to measure the
brain activity in response to those sounds. A normal subject shows a decrease
in brain activity while hearing a second sound, while a subject showing equal
brain activity to the first sound has impaired sensory gating.
Impaired P50 sensory gating is very common in schizophrenia,
also occurs in autism bipolar and even dementia.
There can also be Impaired gating of N100 and P200. The actual definition of these terms gets
complicated and you do not have to go into this level of detail unless you are
really interested
What is N100 event-related potential?
The
N100 is a negative waveform that peaks at approximately 100 milliseconds after
stimulus presentation. Its amplitude is measured using electroencephalography
(EEG) and may be dysfunctional in people with schizophrenia who show an
inability to “gate” or inhibit irrelevant sensory information, ultimately
leading to conscious information overload. To test this, paired auditory clicks
are presented, separated by a short interval, usually of 0.5 seconds. The first
click initiates or conditions the inhibition, while the second (test) click
indexes the strength of the inhibition. An absence of a reduced response to the
second stimulus is interpreted as a failure of inhibitory mechanisms,
postulated to represent a defect in sensory gating.
What is
the evidence for N100 event-related potential?
Moderate
to high quality evidence finds a medium-sized reduction in N100 amplitude to
the first stimulus, but not to the second stimulus. Review authors suggests
this reflects a deficit in processing of auditory salience rather than in
inhibition.
Highlights
·
In the paired-click paradigm, ASD individuals displayed a
significant N100 gating deficit.
·
N100 gating deficit was associated with symptom severity of
sensory sensitivity.
·
P50 and P200 in ASD did not deviate from the typically developing
controls.
·
P50 and P200 were associated with social deficits and attention
switching difficulty in ASD.
We found that compared to TDC, ASD participants had
significant N100 suppression deficits reflected by a larger N100 S2 amplitude,
smaller N100 ratio of S2 over S1, and the difference between the two
amplitudes. N100 S2 amplitude was significantly associated with sensory
sensitivity independent of the diagnosis. Although there was no group
difference in P50 suppression, S1 amplitude was negatively associated with
social deficits in ASD. P200 gating parameters were correlated with attention
switching difficulty. Our
findings suggest N100 gating deficit in adolescents and young adults with ASD.
The relationships between P50 S1 and social deficits and between N100 S2 and
sensory sensitivity warrant further investigation.
Expanding our understanding of sensory gating in children with autism
spectrum disorders
Highlights
·
Children with autism showed significantly reduced gating at P50, N1, and P2
event-related potential components.
·
Children with autism show reduced orientation to auditory stimuli compared
to typically-developing children.
·
Time-frequency analysis show reduced neural synchronization of stimuli in
children with autism.
Abstract
Objective
This study examined sensory
gating in children with autism spectrum disorders (ASD). Gating is usually
examined at the P50 component and rarely at mid- and late-latency components.
Methods
Electroencephalography data
were recorded during a paired-click paradigm, from 18 children with ASD
(5–12 years), and 18 typically-developing (TD) children. Gating was
assessed at the P50, N1, P2, and N2 event-related potential components. Parents
of all participants completed the Short Sensory Profile (SSP).
Results
TD children showed gating at
all components while children with ASD showed gating only at P2 and N2.
Compared to TD children, the ASD group showed significantly reduced gating at P50,
N1, and P2. No group differences were found at N2, suggesting typical N2 gating
in the ASD group. Time-frequency analyses showed reduced orientation and neural
synchronization of auditory stimuli. P50 and N1 gating significantly correlated
with the SSP.
Conclusion
Although children with ASD have
impaired early orientation and filtering of auditory stimuli, they exhibited
gating at P2 and N2 components suggesting use of different gating mechanisms
compared to TD children.
Sensory deficits in ASD may relate to gating.
Significance
The data provide novel evidence for impaired neural orientation, filtering,
and synchronization in children with ASD.
Autism spectrum
disorders (ASD) and schizophrenia are separate disorders, but there is evidence
of conversion or comorbid overlap. The objective of this paper was to explore whether deficits
in sensory gating, as seen in some schizophrenia patients, can also be found in
a group of ASD children compared to neurotypically developed children. An
additional aim was to investigate the possibility of subdividing our ASD sample
based on these gating deficits. In a case–control design, we assessed gating of the P50
and N100 amplitude in 31 ASD children and 39 healthy matched controls (8–12
years) and screened for differences between groups and within the ASD
group. We did not find disturbances in auditory P50 and N100 filtering in the
group of ASD children as a whole, nor did we find abnormal P50 and N100
amplitudes. However, the P50
amplitude to the conditioning stimulus was significantly reduced in the
Asperger subgroup compared to healthy controls. In contrast to what is
usually reported for patients with schizophrenia, we found no evidence for
sensory gating deficits in our group of ASD children taken as a whole. However,
reduced P50 amplitude to
conditioning stimuli was found in the Asperger group, which is similar to what
has been described in some studies in schizophrenia patients. There was
a positive correlation between the P50 amplitude of the conditioning stimuli
and anxiety score in the pervasive developmental disorder not otherwise
specified group, which indicates a relation between anxiety and sensory
registration in this group
Treatments for sensory gating
We know that in schizophrenia impaired P50 gating is associated
with alpha 7 nicotinic acetylcholine receptor (α7 nAChR) dysfunction and shown
to be improved with nicotine and other α7 nAChR agonists.
Other α7 nAChR agonists include:-
·
Acetylcholine
·
Choline
·
Nicotine
·
Tropisetron
Galantamine is a positive allosteric modulator (PAM) of
nAChRs
Why do people with schizophrenia love
to smoke?
A truly remarkable observation is that smoking improves
sensory gating in schizophrenia, but it has the opposite effect on people with
bipolar.
Smoking
as a Common Modulator of Sensory Gating and Reward Learning in Individuals with
Psychotic Disorders
Motivational
and perceptual disturbances co-occur in psychosis and have been linked to
aberrations in reward learning and sensory gating, respectively. Although
traditionally studied independently, when viewed through a predictive coding
framework, these processes can both be linked to dysfunction in striatal
dopaminergic prediction error signaling. This study examined whether reward
learning and sensory gating are correlated in individuals with psychotic
disorders, and whether nicotine—a psychostimulant that amplifies phasic
striatal dopamine firing—is a common modulator of these two processes. We recruited
183 patients with psychotic disorders (79 schizophrenia, 104 psychotic bipolar
disorder) and 129 controls and assessed reward learning (behavioral
probabilistic reward task), sensory gating (P50 event-related potential), and
smoking history. Reward learning and sensory gating were correlated across the
sample. Smoking influenced
reward learning and sensory gating in both patient groups; however, the effects
were in opposite directions. Specifically, smoking was associated with improved
performance in individuals with schizophrenia but impaired performance in
individuals with psychotic bipolar disorder. These findings suggest that
reward learning and sensory gating are linked and modulated by smoking.
However, disorder-specific associations with smoking suggest that nicotine may
expose pathophysiological differences in the architecture and function of
prediction error circuitry in these overlapping yet distinct psychotic
disorders.
When
you look up P50 gating and also Misophonia in the clinical trials database, you
get some Mickey Mouse behavioral treatments for misophonia.
For p50
gating you a decent list of drugs trialed in schizophrenia.
My earlier posts on this subject:-
"I
did wonder how nicotine fits in, since in earlier post we saw that α7 nAChR agonists, like nicotine, improve sensory gating and indeed
that people with schizophrenia tend to be smokers. It turns out that nicotine
is also an HCN channel blocker. For a change, everything seems to fit nicely
together. There are different ways to block HCN channels, some of which are
indirect. One common ADHD drug, Guanfacine, keeps these channels closed, but in a
surprising way."
Abstract
INTRODUCTION:
Sensory gating is a process
involved in early information processing which prevents overstimulation of
higher cortical areas by filtering sensory information. Research has shown that
the process of sensory gating is disrupted in patients suffering from clinical disorders
including attention deficit hyper activity disorder, schizophrenia, and
Alzheimer's disease. Phosphodiesterase (PDE) inhibitors have received an
increased interest as a tool to improve cognitive performance in both animals
and man, including sensory gating.
METHODS:
The current study
investigated the effects of the PDE4 inhibitor Roflumilast in a sensory gating
paradigm in 20 healthy young human volunteers (age range 18-30 years). We
applied a placebo-controlled randomized cross-over design and tested three
doses (100, 300, 1000 μg).
RESULTS:
Results show that Roflumilast
improves sensory gating in healthy young human volunteers only at the 100-μg
dose. The effective dose of 100 μg is five times lower than the clinically
approved dose for the treatment of acute exacerbations in chronic obstructive
pulmonary disease (COPD). No side-effects, such as nausea and emesis, were
observed at this dose. This means Roflumilast shows a beneficial effect on
gating at a dose that had no adverse effects reported following single-dose
administration in the present study.
CONCLUSION:
The PDE4 inhibitor Roflumilast has a favourable side-effect profile at
a cognitively effective dose and could be considered as a treatment in
disorders affected by disrupted sensory gating.
Be wary of antipsychotics!!
Now we see
again that α2A Receptor agonists
like guanfacine and clonidine will improve sensory gating. We should not be
surprised that drugs with the opposite effect (antagonists) will make sensory
gating worse.
α2A
Receptor Antagonists
·
Idazoxan
·
1-PP (active metabolite of buspirone and gepirone, anti-anxiety
drugs)
·
Asenapine
·
BRL-44408
·
Clozapine ,
an anti-psychotic drugs used in schizophrenia
·
Lurasidone an
anti-psychotic drugs used in schizophrenia and in bipolar
·
Mianserin,
an anti-depressant
·
Mirtazapine,
an anti-depressant
·
Paliperidone an
anti-psychotic drugs used in schizophrenia
·
Risperidone,
an anti-psychotic drugs used in schizophrenia and autism
·
Yohimbine
Treatment
for Hyperacusis
If you look
up treatments and trials for hyperacusis (sound sensitivity) you see a list of
cognitive behavioral therapies.
These are
not nonsense. We used something similar to deal with Monty’s extreme aversion
to crying babies when he was young. Now
when he hears a baby crying, he laughs.
But really,
science has much more to offer than behavioral therapy.
I did write many years ago about
hypokalemic sensory overload and its big brother hypokalemic periodic paralysis
(HypoPP). In both conditions it seems
that low levels of potassium cause some pretty severe reactions. Both conditions respond rapidly to an oral
potassium supplement.
Though rare, we know that HypoPP is caused by a dysfunction in the ion
channels Nav1.4 and/or Cav1.1.
For decades one of the treatments for HypoPP has been a diuretic called
Diamox/Acetazolamide. Other treatments
include raising potassium levels using supplements, or potassium
sparing diuretics.
Way back in 2013, I defined a new term, in the post below:-
Hypokalemic Autistic Sensory Overload
I showed an oral potassium supplement
reduced sound sensitivity within 20 minutes, with a simple experiment anyone
can do at home.
Some people do find long term sensory
relief just from the use of an oral potassium supplement once a day. In my son’s case the affect does not last
very long.
Therapies for hypokalemic sensory
overload might be:-
·
A potassium
supplement
·
A potassium
sparing diuretic
·
Possibly Diamox/ Acetazolamide
·
Very likely,
intra-nasal Desmopressin, this lower sodium levels and so will have the
opposite impact on potassium levels
·
Ponstan, the NSAID that affects numerous
potassium ion channels
In some people it appears that Humira,
a long-acting TNF-alpha inhibitor, resolves visual and sound sensitivity. I think this resolves a mixture of
hyperacusis and Misophonia and the visual sensory equivalents.
Tinnitus
Tinnitus is
an extremely common, but is generally regarded as something you just have to
get used to; there are no approved drug therapies.
All kinds of
things can lead to tinnitus. A head injury can lead to tinnitus, exposure to a
loud sound is a common cause, but there is even drug-induced tinnitus. Tinnitus
is a common comorbidity of diabetes.
There is
gradual onset tinnitus and acute onset tinnitus.
Tinnitus is
more likely to occur the older you get and often gets worse over time.
Clearly
there are many sub-types of tinnitus and inevitably there will need to be
multiple different therapies
Full graphic is available at fnins-13-00802-g004.jpg
(4660×2924) (frontiersin.org)
The paper below is
very comprehensive:
Tinnitus is unusual for such a common symptom
in that there are few treatment options and those that are available are aimed
at reducing the impact rather than specifically addressing the tinnitus
percept. In particular, there is no drug recommended specifically for the
management of tinnitus. Whilst some of the currently available interventions
are effective at improving quality of life and reducing tinnitus-associated
psychological distress, most show little if any effect on the primary symptom
of subjective tinnitus loudness. Studies of the delivery of tinnitus services
have demonstrated considerable end-user dissatisfaction and a marked disconnect
between the aims of healthcare providers and those of tinnitus patients:
patients want their tinnitus loudness reduced and would prefer a
pharmacological solution over other modalities. Several studies have shown that
tinnitus confers a significant financial burden on healthcare systems and an
even greater economic impact on society as a whole. Market research has
demonstrated a strong commercial opportunity for an effective pharmacological treatment
for tinnitus, but the amount of tinnitus research and financial investment is
small compared to other chronic health conditions. There is no single reason
for this situation, but rather a series of impediments: tinnitus prevalence is
unclear with published figures varying from 5.1 to 42.7%; there is a lack of a
clear tinnitus definition and there are multiple subtypes of tinnitus,
potentially requiring different treatments; there is a dearth of biomarkers and
objective measures for tinnitus; treatment research is associated with a very
large placebo effect; the pathophysiology of tinnitus is unclear; animal models
are available but research in animals frequently fails to correlate with human
studies; there is no clear definition of what constitutes meaningful change or
“cure”; the pharmaceutical industry cannot see a clear pathway to distribute
their products as many tinnitus clinicians are non-prescribing audiologists. To
try and clarify this situation, highlight important areas for research and prevent
wasteful duplication of effort, the British Tinnitus Association (BTA) has
developed a Map of Tinnitus. This is a repository of evidence-based tinnitus
knowledge, designed to be free to access, intuitive, easy to use, adaptable and
expandable.
The next paper makes the key point
that to treat tinnitus you need precision (personalized) medicine and apply the
neuroscience.
Towards a Mechanistic-Driven Precision Medicine Approach for Tinnitus
In this
position review, we propose to establish a path for replacing the empirical
classification of tinnitus with a taxonomy from precision medicine. The goal of a
classification system is to understand the inherent heterogeneity of
individuals experiencing and suffering from tinnitus and to identify what
differentiates potential subgroups. Identification of different patient
subgroups with distinct audiological, psychophysical, and neurophysiological characteristics
will facilitate the management of patients with tinnitus as well as the design
and execution of drug development and clinical trials, which, for the most
part, have not yielded conclusive results. An alternative outcome of a
precision medicine approach in tinnitus would be that additional mechanistic
phenotyping might not lead to the identification of distinct drivers in each
individual, but instead, it might reveal that each individual may display a
quantitative blend of causal factors. Therefore, a precision medicine approach
towards identifying these causal factors might not lead to subtyping these
patients but may instead highlight causal pathways that can be manipulated for
therapeutic gain. These two outcomes are not mutually exclusive, and no matter
what the final outcome is, a mechanistic-driven precision medicine approach is
a win-win approach for advancing tinnitus research and treatment. Although
there are several controversies and inconsistencies in the tinnitus field,
which will not be discussed here, we will give a few examples, as to how the
field can move forward by exploring the major neurophysiological tinnitus
models, mostly by taking advantage of the common features supported by all of
the models. Our position
stems from the central concept that, as a field, we can and must do more to
bring studies of mechanisms into the realm of neuroscience.
I did have a quick look the clinical
trials website to see if there have been any interesting trials that did show
some benefit.
I noted the following drugs:
Lidocaine
Lidocaine, the anesthetic that targets
sodium ion channels. Careful titration allows for a high
degree of selectivity in the blockage of sensory neurons. This looks like a good idea. Originally, they
played with intravenous delivery, but then moved no to transdermal.
In this preliminary study, 5% transdermal
lidocaine appears to be a potential treatment for chronic subjective tinnitus.
The majority of subjects who completed 1 month of treatment had clinically
significantly improved tinnitus. These findings are confounded however by the
small sample size and significant drop out rate.
Clonazepam
Clonazepam
is a benzodiazepine drug that activates GABAa receptors. The trials are a bit mixed and one showed it
only worked when given together with Deanxit. Deanxit is a combination of Flupentixol, an
antipsychotic, and melitracen an tricyclic antidepressant.
These
look like bad options which will end up causing new problems over time.
Clonazepam Quiets tinnitus: a randomised
crossover study with Ginkgo Biloba
Conclusion Clonazepam
is effective in treating tinnitus; G biloba is ineffective.
Results: Significant tinnitus reduction was seen
after intake of the combination clonazepam-Deanxit, whereas no differences in
tinnitus could be demonstrated after the administration of clonazepam-placebo.
This was true for all patients according to the following parameters: time
patients are annoyed by the tinnitus (p = 0.026) and the visual analogue scale
for tinnitus annoyance (p = 0.024).
Conclusion: Although tinnitus reduction was recorded as
modest, this article provides valuable data demonstrating a placebo-controlled
tinnitus reduction after clonazepam and Deanxit intake.
Oxytocin
There already is a lot in the blog
about oxytocin and I was surprised anyone had trialed it for tinnitus, but they
did and it seems to provide a benefit.
As regular readers of this blog know, there looks to be a better way to
deliver oxytocin to the brain than intra-nasal. We saw how a specific gut
bacteria has the same effect (Biogaia Protectis).
Conclusion
These preliminary
studies demonstrated that oxytocin may represent a helpful tool for treating
tinnitus and further larger controlled studies are warranted.
Acamprosate
Acamprosate is used to treat
alcoholics.
“An
inhibition of the GABA-B system is
believed to cause indirect enhancement of GABAA receptors.[17] The
effects on the NMDA complex are dose-dependent; the product appears to enhance
receptor activation at low concentrations, while inhibiting it when consumed in
higher amounts, which counters the excessive activation of NMDA receptors in
the context of alcohol withdrawal”
Objectives: Tinnitus is a
common and distressing otologic symptom, with various probable pathophysiologic
mechanisms, such as an imbalance between excitatory and inhibitory mechanisms.
Acamprosate, generally used to treat alcoholism, is a glutaminergic antagonist
and GABA agonist suggested for treating tinnitus. Thus, we aimed to evaluate
the efficacy and safety of acamprosate in the treatment of tinnitus.
Conclusions: The study
results indicated a subjective relief of tinnitus as well as some degree of the
electrophysiological improvement at the level of the cochlear and the distal
portion of the auditory nerve among the subjects who received the acamprosate.
Magnesium
Magnesium
supplementation, being cheap and OTC, is a common therapy for tinnitus. It does seem to provide a benefit for some.
Conclusion: The results
suggest that magnesium may have a beneficial effect on perception of
tinnitus-related handicap when scored with the THI.
Neramexane
Neramexane is interesting because it is closely related to
Memantine/Namenda, which was widely used in autism, but failed in its large
clinical trial. Memantine is seen as an
NMDA receptor antagonist/blocker, but it also blocks nicotinic
acetylcholine receptors (nAChRs) which play a role in Alzheimer’s
and sensory gating (Misophonia). Memantine also affects serotonin and dopamine
receptors.
Neramexane is a new
drug being developed for Alzheimer’s and as a pain killer.
Neramexane is a new substance that exhibits
antagonistic properties at α9α10 cholinergic
nicotinic receptors and N-methyl-D-aspartate receptors, suggesting potential
efficacy in the treatment of tinnitus.
Conclusions
This study
demonstrated the safety and tolerability of neramexane treatment in patients
with moderate to severe tinnitus. The primary efficacy variable showed a trend
towards improvement of tinnitus suffering in the medium- and high-dose
neramexane groups. This finding is in line with consistent beneficial effects
observed in secondary assessment variables. These results allow appropriate
dose selection for further studies.
Mirtazapine
Mirtazapine is yet another drug that
has been covered in this blog. It is a
very cheap anti-histamine / anti-depressant.
We saw in this blog that the effect is
highly dose dependent. It affects very
many receptors and the overall effect depends on dosage. The antidepressant
effect is at the dose of 15+mg. In this person
with tinnitus, they used 7.5mg. For some conditions the dose goes up to 60mg a
day.
At very low dosages mirtazapine is a
potent H1 anti-histamine and makes you very drowsy
One parent noted that low dose Mirtazapine
had a highly beneficial effect in their child with autism.
Auditory pathways are
modulated by various neurotransmitters such as serotonin responsible for sound
detection, location, and interpretation. The neurotransmitter gamma amino
butyric acid (GABA) is inhibitory in the auditory system. Given that there is
preferential innervation of the GABAergic neurons in the inferior colliculus by
serotonergic neurons, it may be plausible then that antidepressant drugs, by
increasing the availability of serotonin and thereby increasing GABAergic
activity, provide relief from the symptoms of tinnitus.5 This report shows
that mirtazapine may have a beneficial effect in the subgroup of patients
suffering from tinnitus but exact mechanism is difficult to put forward.
Conclusion
I think we are absolutely spoilt for
choice.
So many possible therapies, each one
effective in some cases.
The key is precision medicine, personalized to the individual case in question. This approach was also proposed in the recent paper on Tinnitus, only without telling us what to actually do!
In my son, now 18 with what we can
call treated severe autism, the clear winner so far is Ponstan (Mefenamic
Acid). Diclofen, a very common Fenamate
class drug, does share the same effect, but to a lesser extent.
Low dose Roflumilast, the P50 sensory gating therapy (that is more for Aspies) has no sensory effect at all. It is the same dose as that proposed in the research to raise IQ.
The intranasal Desmopressin mentioned
by one reader is another good choice to consider, but you may need to
supplement sodium. I think if you get a short term benefit from a 500mg potassium supplement, this is worth a try.
For Aspies low dose Roflumilast
everyday looks worth a try, while Humira every 2 months look interesting, but it will
be hard to get and is pricey.
For people with Schizophrenia, they
could look at tobacco alternatives, which would include low-dose Roflumilast.
People with Bipolar might want to look
at Mirtazapine – the opposite of nicotine and which also helps some cases of
tinnitus.
For tinnitus I thought oxytocin looked
a very safe option. You have intranasal,
or my preference the gut bacteria probiotic that stimulates oxytocin release in the
brain.
Magnesium is a safe bet for
tinnitus. Transdermal lidocaine makes
sense, but is a bit more daring. Memantine might be worth a shot, if nothing
else helps.
You can also increase sound and visual
sensitivity. Low dose DMF (dimethyl fumarate) increases sound sensitivity and
the TRH super-agonist Ceredist increases visual sensitivity. For most people with autism, you likely do
not need either effect.